It's all about methylation: everything you didn't know, you need to know, about methylation
and why potentially half of the readership here really does need to know about this.
I am unvaxxed.
The information provided on this article does not, and is not intended to, constitute medical advice; instead, all information, content, and materials available on this site are for general informational purposes only.
Introduction to the Article
This article is intended as a reference guide, so bookmark it and return when you need the information provided here. It’s designed for easy skim reading:
Headings: Read all the Heading 1s and Heading 2s for a general overview of the article.
Lead Sentences: Check the lead sentences under each heading for summaries of the content under that heading.
Exploration: If you choose to explore the MTHFR gene for yourself or a loved one, you will eventually want to read the entire article as a primer - but only as a primer. You will have to do a lot of personal research to apply it to yourself. For those with the MTHFR variants, this is just the beginning of what I hope will be a long and rewarding journey toward real people learning to manage our own health in the 21st century. Bold claim? Maybe!
Table of Contents
Part 3 - The Critical Role of Folate (B9) in the Methylation Process
Part 5 - How to Ensure Sufficient Methyl Donors for Optimal Health
Part 7 - The Role of the COMT Gene in the Methylation Process
Part 8 - The Role of the MTHFR and COMT Genes in Histamine Reactions
Part 9 - The Role of DNA Methylation in the Regulation of our Genes
Part 10 - Where to Start with Fixing Ill Health Caused by Poor Methylation
Part 1 - What is the MTHFR Gene
I have only recently become aware of the MTHFR gene, and it has put a bomb under everything I thought I knew about my own health. It has blown sky high every failed regimen imposed upon me by doctors and naturopaths alike. I can happily say that any medical practitioner of any persuasion, who is not starting their role as your health care advisor by working with you to study your genes, is irresponsible, and a waste of your time and money. Bold claims.
I only recently discovered that I have a variant of the MTHFR gene, and in learning about this gene, I learned how it has contributed to both my ongoing lifelong battle with fibromyalgia, and my recent serious and potentially terminal health collapse, post hospitalisation, with what was called “covid”.
Since I discovered the MTHFR gene, I’ve dived into all sorts of materials about the gene and joined a few Facebook groups designed to support those who have these genetic variants.
I usually expect to understand new ideas relatively easily, but this topic has really tested me. The confusion I saw in those Facebook groups made it clear that others are struggling too. So, here’s my shot at making this complex information more understandable for anyone affected. I want this guide to be a starting point for anyone who decides to manage the health aspects tied to this gene—because trust me, it is manageable once you realize you need to tackle it!
It’s essential we understand the importance of the MTHFR gene, because about 50% of the population carries a variant that can lead to impaired health. This could explain why so many of us are feeling unwell in our heavily polluted world. We know that industrial activities have made our environment toxic, yet some people suffer while others seem to be unaffected. Why is that?
Genetics is the key!
In this article, I’m focusing on just one gene out of the thousands we carry, but it’s amazing how a single gene can influence a large part of the health of a large part of our population.
There are a lot of new terms thrown around when discussing this topic, so I will try to make sure I define all my terms, and use them consistently, as I go.
Part 2 - What is Methylation?
The MTHFR Gene, the MTHFR Enzyme, and Methyl Groups
The MTHFR gene provides the instructions for creating an enzyme called methylenetetrahydrofolate reductase (the MTHFR enzyme). This enzyme plays a crucial role in a chemical reaction that transforms folate into its active form, 5-methyltetrahydrofolate (5-MTHF).
Folate has no methyl groups, but once converted into 5-MTHF, each molecule carries one methyl group.
So, what exactly is a methyl group? It consists of four atoms—one carbon atom and three hydrogen atoms (CH₃). Once we have 5-MTHF, this active form of folate can become a methyl donor, and donate its methyl group to support various essential functions in our bodies, through a process known as methylation.
Methylation
Methylation is all about transferring a methyl group (CH₃) from one substance to another in the body. It drives many critical and complex chemical processes, and it is essential for several vital functions, including:
Cardiovascular and immune system activity
Neurotransmitter production
Hormone regulation
Energy production
Heavy metal detoxification
DNA repair and gene expression.
Our bodies have a constant high demand for methyl groups as methylation supports so many crucial processes. However, we can only store a limited amount of methyl groups, so we need a steady supply. This supply comes from our diet and from our body's internal methylation cycle during which we can both create and recycle methyl groups.
However, not all bodies are equally effective at creating and recycling methyl groups.
Variants of the MTHFR Gene and Their Impact on Methylation
Certain variants of the MTHFR gene, such as the C677T and A1298C variants, can reduce the availability of the MTHFR enzyme, and so reduce the body’s capacity to convert folate to its active form, 5-MTHF.
Individuals with these MTHFR variants only have 20% to 80% of the normal capacity for methylation. But here's the good news: everyone who has survived pregnancy can still produce at least some MTHFR enzyme, allowing us all to complete a portion of the methylation process.
Methylation needs are unique to each person, influenced by factors such as gender, race, age, as well as genetic, hormonal, and environmental influences. Because of this variability, there’s no “one size fits all” method to determine or measure someone’s optimal methylation capacity or baseline. Each person’s needs are distinct, and these factors, along with genetic variants like MTHFR, shape how much methylation support each individual might require.
Part 3 - The Critical Role of Folate (B9) in the Methylation Process
The Impact of MTHFR Gene Variants on Our Ability to Use Dietary Folate
Understanding how our body processes dietary folate (B9) is crucial, especially if we have MTHFR gene variants that limit our ability to process this important vitamin. If we do not consume enough of the right form of folate (B9) we might find ourselves either deficient in methyl groups or battling the potentially toxic effects of the wrong form of folate.
Types of Folate
Here’s a breakdown of the various forms of B9 (folate) and how they differ. The key distinction lies in how easily each form can be metabolized and used, especially for those with compromised MTHFR function.
Folate (Natural): This form comes from foods like leafy greens. Our bodies need to convert it into 5-MTHF to use it effectively. However, with MTHFR genetic variants, we may not be able to methylate the folate, and so we will excrete it, unused. Luckily, since folate is water-soluble, any excess that can’t be processed isn’t toxic. So, while that kale and spinach smoothie might not be doing much for us, it won’t harm us either.
Folinic Acid (5-Formyl THF): This is an active, naturally occurring form found in the same foods as folate. It bypasses some conversion steps, making it easier for the body to use, but it’s not available in high enough amounts to fully replace folate.
5-MTHF (5-Methyltetrahydrofolate): This is the fully active form that the body can use right away. It’s only found in small amounts in foods, making supplementation a preferred choice for those with MTHFR variants.
Folic Acid: This is the synthetic form found in supplements and fortified foods. The body needs to convert it to 5-MTHF before it can enter the methylation cycle.
Many Western countries have legislated the addition of folic acid to “white” wheat and rice products, cereals and baby foods. You will find it in almost all processed foods.
However, people with MTHFR gene variants can struggle to convert folic acid efficiently. This can lead to unmetabolized folic acid building up, and blocking the folate receptors. If it is not processed, it sits there for days, weeks or even months until the receptor it is occupying dies, both are removed from the body, and new receptors are produced. Meanwhile natural folate is sitting in the queue waiting to be processed into a methylated form, and there are no free receptors for it. Traffic jam, and the body is not getting the methylated folate it needs down the line. The long-term effects of this are unknown but might include:
Masking Vitamin B12 Deficiency
Immune Function Suppression
Cancer Risks
Neurological Concerns
Potential for Epigenetic Changes
Until more research clarifies these risks, it’s best to avoid foods “fortified” with folic acid, and supplements that contain folic acid. Even high-quality B complex vitamins may contain folic acid, so be sure to check those labels!
Part 4 - The Effects of Inadequate Methylation
There are many health risks that can result from inadequate levels of methyl groups, across a wide range of bodily functions.
1. Elevated Homocysteine Levels
Poor methylation can lead to elevated homocysteine levels, a condition known as hyperhomocysteinemia. This can have several negative effects on health:
Cardiovascular Issues: High homocysteine levels can contribute to atherosclerosis, heart attacks, strokes, damage to the endothelium (the inner lining of blood vessels), inflammation, and thrombosis.
Neurological Effects: Elevated homocysteine may affect neurotransmitter production and neuronal health, increasing the risk of neurodegenerative diseases like Alzheimer’s disease and cognitive decline.
Increased Oxidative Stress: This can lead to cellular damage and inflammation.
2. Impaired DNA Methylation
Gene Expression: Methylation plays a vital role in regulating gene expression. A lack of methyl donors can cause hypomethylation (too little methylation) or hypermethylation (too much methylation), disrupting normal cellular functions and potentially contributing to diseases like cancer.
Epigenetic Changes: Alterations in DNA methylation can affect how genes are expressed, influencing processes like cell specialization, growth, and even increasing the risk of certain diseases.
3. Neurotransmitter Imbalances
Mental Health Issues: Methylation is crucial for synthesizing neurotransmitters like serotonin, dopamine, and norepinephrine. Insufficient methyl donors can impair neurotransmitter production, leading to mood disorders, anxiety, and cognitive problems.
Neurological Disorders: Low methylation levels are linked to a higher risk of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s.
4. Hormonal Imbalances
Methylation is also involved in hormone regulation. A deficiency in methyl donors can disrupt the synthesis and metabolism of hormones, potentially leading to endocrine disorders.
5. Impaired Detoxification
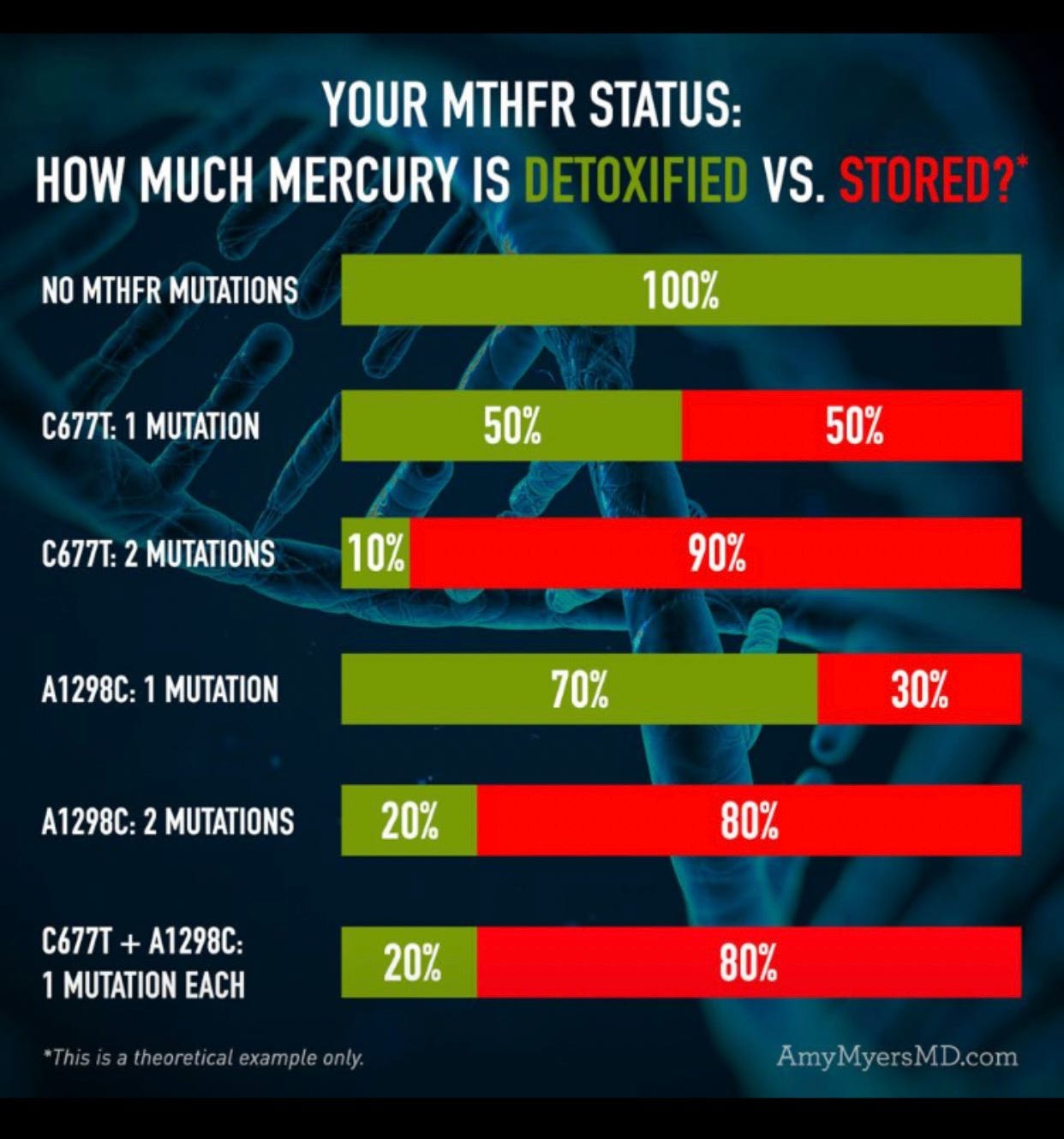
Accumulation of Toxins: Methylation is essential for detoxifying harmful substances in the liver. Without enough methyl donors, the body may struggle to process and eliminate toxins, heavy metals, and certain drugs. One example of this is shown below in relation to the MTHFR variants and the ability to detoxify mercury.
Increased Toxicity: Inadequate methylation can lead to an accumulation of toxic metabolites, potentially causing oxidative stress and cellular damage.
6. Cardiovascular Health
Insufficient methyl donors can also affect lipid metabolism, leading to dyslipidemia (abnormal lipid levels) and increasing the risk of cardiovascular diseases.
7. Increased Oxidative Stress
Methylation plays a role in producing antioxidants like glutathione. Inadequate methyl donors can reduce glutathione levels, resulting in increased oxidative stress and cellular damage.
We can reduce these risks by paying attention to our overall consumption and production of methyl donors and methyl groups.
Part 5 - How to Ensure Sufficient Methyl Donors for Optimal Health
Our bodies have a nearly continuous need for methyl donors that can supply methyl groups. We can obtain these through our diet, supplements, and our body's ability to manufacture them from precursors, or recycle them. Here are four primary sources of methyl groups:
Source 1 - We Can Eat Methyl Donors
We ingest approximately 50 mmol of methyl groups per day from our diet. However, methyl groups from different dietary sources serve different functions in the body, meaning one does not directly replace another. Here are our food sources of methyl donors:
Betaine: Mostly supplied by beets, spinach, and whole grains (such as quinoa, wheat and oat brans, brown rice, and barley). Also known as trimethylglycine (TMG), naturally contains three methyl groups, known as betaine derived methyl groups. Foods rich in betaine can be more concentrated sources of methyl donors compared to choline-rich foods, making them efficient dietary options for meeting methylation needs. When consumed, betaine can donate one of its methyl groups in the homocysteine-to-methionine conversion, contributing to vital methylation processes. As we have seen in the previous section, without sufficient methyl donors, excess homocysteine can accumulate in the blood, potentially causing harmful reactions and making it difficult for the body to dispose of.
Choline: Found in choline-rich foods such as eggs, liver, and soy, choline can provide up to 60% of our dietary methyl groups. Choline contains three methyl groups in its structure, all available for donation, known as choline-derived methyl groups. Choline can also be converted into betaine, which then acts as a methyl donor in the homocysteine-to-methionine conversion pathway. This conversion allows for more efficient utilization of methyl groups in specific metabolic pathways, particularly in homocysteine metabolism.
Methionine: This amino acid, present in protein-rich foods like nuts, beef, lamb, cheese, turkey, pork, fish, shellfish, soy, eggs, dairy, and beans, can be consumed directly or converted through other methylation pathways. Once ingested, methionine is converted into S-adenosylmethionine (SAMe) through a reaction with adenosine triphosphate (ATP). SAMe is a key methyl donor in many biological methylation reactions, contributing to DNA Methylation,
Neurotransmitter Synthesis, and Lipid Metabolism. The chemistry involved in this process can become complex, so those interested in deepening their understanding of these methylation pathways can explore this further, independently.
Folate and Methylated Folate: Small amounts of methylated folate are found in foods rich in natural folate, especially dark green leafy vegetables, fruits, fruit juices, nuts, beans, peas, seafood, eggs, dairy products, meat, poultry, and grains. However, methylated folate is present only in tiny amounts and may not compensate for significant conversion difficulties from folate to 5-MTHF.
Source 2 - We Can Manufacture Methyl Groups
It is unlikely that we can rely solely on dietary intake for methyl groups, especially in a typical Western diet, so our body has the capacity to manufacture them also. We convert non-methylated folate (B9) from our diet into its active, methylated form (5-MTHF) thanks to the MTHFR enzyme, produced by the MTHFR gene.
We are battling two issues when it comes to getting enough 5-MTHF into our bodies:
The standard western processed food diets have very low levels of usable folate, meaning that they are likely to provide less than 30% of the requirement for methyl donors. Even fresh, whole food diets can provide only 60 to 90% of needed methyl groups, as these foods have a higher content of bioavailable methyl donors like 5-MTHF, choline, betaine, and methionine. But no diets are likely to supply 100% of needs.
Our body is designed to make up for this shortfall in part by being able to manufacture methyl groups as required using the MTHF enzyme to process dietary folate. However, as we already know, those with certain critical MTHFR gene variants are not able to do this efficiently.
Source 3 - We Can Supplement with Methyl Donors
When dietary sources are insufficient, supplementation can help bridge the gap. Effective supplements include:
S-Adenosylmethionine (SAMe)
5-MTHF (Methylated Folate): 5-MTHF is the biologically active form of folate, bypassing the need for conversion via the MTHFR enzyme.
Methylcobalamin: The methylated form of vitamin B12.
Betaine (Trimethylglycine or TMG).
Each of these supplements has potential downsides based on individual genetic makeup, so supplementation should be carefully planned and monitored.
Source 4 - We Can Recycle Methyl Groups
Some methyl groups can be recycled from one function to another, which is particularly important when methylation is compromised. At this stage, the chemistry involved can become complex, but those interested in deepening their understanding of methylation and the MTHFR gene can explore this further, independently.
Part 6 - The Role of B12 in the Methylation Process
While this article has primarily focused on the methylation of vitamin B9, vitamin B12 plays a crucial and complex role in this process. Understanding how B12 fits into the methylation cycle is important, and it will be different for each of us.
The Importance of Vitamin B12
Vitamin B12 is essential for the methylation cycle, helping the body create vital compounds like DNA, proteins, and neurotransmitters. Specifically, B12 works hand-in-hand with folate to convert homocysteine—a potentially harmful amino acid—into methionine. Methionine is critical for producing S-adenosylmethionine (SAMe), which serves as the body's primary methyl donor. This process is vital for regulating gene expression, detoxification, and energy production.
The Best Form of B12 for MTHFR Variants
Vitamin B12 comes in several forms, each with slightly different roles in the body:
Methylcobalamin: This is the active form of B12 that directly participates in the methylation cycle. It helps convert homocysteine into methionine and is particularly recommended for those with MTHFR variants, as it's already in the active form that the body can use directly in the methylation cycle.
Adenosylcobalamin: This active form mainly functions in the mitochondria (the energy-producing parts of cells). While it's important for energy metabolism and overall cellular health, it is less directly involved in the methylation cycle.
Hydroxocobalamin: Often used in injectable form, hydroxocobalamin can be converted into both methylcobalamin and adenosylcobalamin in the body. It's a more flexible form of B12 that can convert into different types and may benefit those with MTHFR variants.
Cyanocobalamin: This synthetic form is commonly found in many supplements. However, it requires conversion into active forms (methylcobalamin or adenosylcobalamin), which may be less efficient for individuals with MTHFR variants. It’s generally best avoided for those with these variants.
Part 7 - The Role of the COMT Gene in the Methylation Process
Another critical gene for those with the MTHFR variants is the COMT gene. The activity level of COMT can significantly impact overall methylation and various metabolic processes.
The COMT enzyme (catechol-O-methyltransferase) plays a crucial role in the methylation process, particularly in metabolizing catecholamines like dopamine, norepinephrine, and epinephrine, as well as other catechol compounds.
Impact of Slow COMT on Methylation
Individuals with slow COMT activity—often due to specific genetic variants like the COMT rs4680 variant—may struggle to effectively methylate catecholamines. This can lead to:
Increased Levels of Catecholamines: There can be a buildup of neurotransmitters like dopamine and norepinephrine that can can contribute to anxiety, stress responses, and other mental health issues.
Altered Methylation Capacity and Hypermethylation: There can also be a bottleneck of excess methyl donors that, whilst being required by the body, cannot be used. This may cause hypermethylation which is the excessive addition of methyl groups to DNA or other molecules, which can lead to gene silencing, altered gene expression, and potential disruptions in cellular function.
Potential Deficits in Other Methylation Processes: Slow COMT can impair the overall availability of methyl groups, which might affect other methylation pathways, including those involved in DNA methylation and the metabolism of homocysteine and methionine.
There are some trial-and-error methods for managing slow COMT, including consuming appropriate methyl donor foods and possibly supplements in small amounts throughout the day, rather than having large nutritious meals, or large doses of methylated supplements, that could lead to overmethylation. If you have both an MTHFR gene variant and slow COMT, fasting or intermittent fasting may not be ideal for you. Browsing may help manage methyl group bottlenecks caused by slow COMT.
Optimize the Dose of Methyl Donors
Low, Balanced Supplementation: Instead of taking high doses of methyl donors (like methylfolate or methylcobalamin), start with lower, more balanced doses. Spread them out throughout the day to avoid overwhelming the system.
Rotate Methyl Donors: Use a mix of different sources such as betaine (trimethylglycine), phosphatidylcholine, and folate to prevent over-reliance on any single methyl donor, ensuring broader methyl group distribution.
Support COMT Function Indirectly
Magnesium: COMT requires magnesium to function optimally. Ensure you’re getting enough magnesium, as it can enhance COMT’s ability to metabolize catecholamines even if it’s slower genetically. Magnesium glycinate is often recommended because it is well-absorbed and gentle on the digestive system, making it a good choice for long-term use. Spreading magnesium supplementation throughout the day can help maintain stable levels and avoid overwhelming the body with a large dose at once. Taking magnesium with food can enhance absorption and minimize the potential for digestive upset, though some people find it beneficial to take it in the evening as it may promote relaxation and improve sleep quality. For slow COMT, consistent intake is key, so splitting doses morning and evening may be ideal.
Lithium Orotate (Low Dose): Some people use low-dose lithium orotate to support COMT function, helping with neurotransmitter balance, but only under guidance, as lithium affects mood and nervous system function and functions as a drug rather than a food.
Regulate Dopamine Levels
Dopamine-Modulating Nutrients: B vitamins (like B6) help regulate dopamine synthesis and breakdown. Supporting these pathways can balance neurotransmitter levels and reduce strain on COMT.
Lifestyle Adjustments: Activities that help stabilize dopamine production, such as exercise, regular sleep, and stress management, can reduce the need for COMT to work as hard in metabolizing excess catecholamines.
Incorporate Antioxidants and Detox Support
Glutathione Support: A well-functioning glutathione system helps detoxify the byproducts of methylation and supports overall methylation balance. Nutrients like N-acetylcysteine (NAC) or liposomal glutathione can help.
Methylation Bypass: In cases where methylation builds up too much, antioxidants like vitamin C and E or glutathione may provide some relief by neutralizing oxidative stress, helping cells manage excess methylation.
Monitor with Testing
Monitor Homocysteine Levels: Elevated homocysteine can indicate that methylation is either underactive or blocked, signaling a need to adjust your methyl donors. You can use this as a biomarker to fine-tune your methylation balance.
Track Neurotransmitter Levels: Some people choose to test their dopamine, norepinephrine, and epinephrine levels to see if slow COMT is causing excess neurotransmitter buildup. This can guide how much to limit or adjust methyl donors.
Cofactors to Support Methylation Efficiency
Ensure adequate intake of B2 (riboflavin), B3 (niacin), and B6 (pyridoxine), as these are essential in the methylation cycle and can help make the process more efficient without creating a bottleneck with excess methyl groups.
Impact of Fast COMT on Methylation
Individuals with fast COMT activity are generally more efficient at metabolizing catecholamines, leading to different outcomes:
Lower Levels of Catecholamines: Faster breakdown of neurotransmitters can lead to a more stable mood and reduced anxiety. However, it might also result in insufficient neurotransmitter levels, in some cases.
Balanced Methylation Capacity: Faster COMT activity helps maintain balance in the methylation cycle, preventing substrate buildup and allowing for effective methylation of DNA and other compounds.
Efficient Utilization of Methyl Donors: Individuals with fast COMT may utilize available methyl donors from their diet and the methylation cycle more efficiently, benefiting various methylation-dependent processes.
Broader Impact Beyond Folate Methylation
The activity of COMT influences more than just folate methylation; it also affects several critical aspects of methylation, including:
Hormone Regulation: Methylation pathways are essential for synthesizing and degrading hormones. Altered COMT activity can disrupt hormonal balance.
Detoxification: Effective methylation is crucial for detoxifying various compounds, and COMT activity plays a significant role in overall detoxification processes.
DNA Methylation: Methylation of DNA is vital for gene regulation, and changes in COMT activity can indirectly influence this process by affecting the availability of methyl groups.
In summary, whether you have slow or fast COMT activity can significantly impact your methylation cycle, affecting neurotransmitter metabolism, hormonal regulation, detoxification processes, and DNA methylation. Understanding your COMT status can help tailor dietary and lifestyle interventions to support optimal methylation and enhance your overall health.
Part 8 - The Role of the MTHFR and COMT Genes in Histamine Reactions
1. Histamine Metabolism and Methylation
Histamine, a compound involved in immune responses, allergies, and gastric acid regulation, needs to be broken down in the body to prevent excessive reactions (e.g., allergies, inflammation). The body primarily uses two enzymes to break down histamine:
Histamine N-methyltransferase (HNMT): This enzyme breaks down histamine in the central nervous system and other tissues by adding a methyl group to histamine, a process dependent on methylation.
Diamine Oxidase (DAO): This enzyme is responsible for breaking down histamine in the digestive system, but it’s less directly dependent on methylation.
If the methylation cycle is impaired (e.g., due to an MTHFR gene mutation), your body may have a reduced ability to add methyl groups to histamine through HNMT, slowing histamine breakdown. This can lead to histamine intolerance or an overactive histamine response, causing symptoms such as:
Allergies
Flushing
Hives
Headaches
Digestive issues.
2. MTHFR, COMT, and Histamine
MTHFR Variants: People with MTHFR variants may have a limited capacity to produce methyl groups, which are crucial for histamine breakdown via the HNMT enzyme. This can exacerbate histamine-related symptoms.
COMT Variants: The catechol-O-methyltransferase (COMT) enzyme also uses methyl groups to break down certain neurotransmitters (like dopamine and norepinephrine), and if COMT is slow (as in some genetic variants), it competes with HNMT for available methyl groups. This competition can further reduce the body’s ability to metabolize histamine efficiently.
3. High Histamine and Methyl Donor Depletion
Histamine breakdown via HNMT uses up methyl groups, which are also needed for other critical processes like DNA methylation and detoxification. If histamine levels are consistently high, it can deplete available methyl donors, contributing to methylation imbalances. This may lead to a cycle of methylation stress where the body can’t keep up with the demand for methyl groups, worsening both histamine intolerance and other symptoms related to poor methylation.
4. Impact of Diet on Histamine and Methylation
Certain foods high in histamine (e.g., fermented foods, alcohol, aged cheeses) can further increase histamine levels, putting more strain on the methylation cycle. If methylation is already compromised (due to an MTHFR variant, for example), this can lead to worsened symptoms. In these cases, dietary strategies that both support methylation and manage histamine levels are essential.
5. Supporting Histamine Metabolism with Methylation Support
People with both methylation issues and histamine intolerance can benefit from supporting methylation through:
Methyl donors like 5-MTHF (active folate), methylcobalamin (B12), and betaine (TMG).
DAO supplements to help break down histamine in the gut.
Low-histamine diets to reduce the load on the body’s histamine-metabolizing pathways.
Magnesium and B6, which are important for both methylation and DAO function.
Conclusion
Histamine reactions and methylation are closely linked due to the role of methylation in breaking down histamine. Impaired methylation, often due to MTHFR or COMT variants, can lead to reduced histamine metabolism, contributing to histamine intolerance and related symptoms. Supporting methylation pathways can improve the body's ability to handle histamine and may help alleviate histamine-related reactions.
Part 9 - The Role of DNA Methylation in the Regulation of our Genes
While our genes are inherited and remain fixed, how those genes are expressed—or turned on and off—can be regulated through a process called DNA methylation.
Turning Genes On and Off
Although we inherit genes from our parents, not all genes are active at all times. DNA methylation adds a methyl group to specific parts of the DNA, particularly at the promoter region of a gene. This addition can either silence (turn off) a gene or activate (turn on) it.
Heavily Methylated Genes: If a gene is heavily methylated, it may be ‘turned off,’ meaning its instructions won't be used to make proteins.
Unmethylated Genes: Conversely, if the gene is not methylated, it may be ‘turned on’, allowing the body to utilize that gene's information.
Gene Expression Control
The expression of genes—essentially, how much of a particular protein is made—can be influenced by various factors, including environmental influences (like diet, stress, and toxins) and lifestyle choices. DNA methylation is one way that cells respond to these factors, allowing them to adapt to changes in their environment.
This means that even though you inherit a fixed set of genes, the way your body uses those genes can change based on your experiences and surroundings.
Development and Differentiation
During development, different cells in your body need to utilize different sets of genes. For instance, a skin cell and a liver cell contain the same DNA, but perform very different functions. DNA methylation helps regulate which genes are active in each cell type, guiding cells to develop into specific types and fulfill their unique roles.
In summary, while our genetic code is fixed, DNA methylation is a crucial process that controls which genes are turned on or off. This regulation allows our bodies to adapt to varying conditions, manage development, and respond to environmental changes. It plays an essential role in how our inherited genes function in everyday life!
Part 10 - Where to Start with Fixing Ill Health Caused by Poor Methylation
You can’t change your genes, but you can manage them to minimize the impact of genetic variants on your health. This journey is complex and individualized, so here’s where you might want to start:
1. Get Educated
Join some Facebook MTHFR support groups. These can provide immediate insights into the complexities of managing MTHFR and methylation issues, and prompt important questions you might not have considered.
Consider listening to, watching, or reading material from a few key experts. Here are a few names to check out. They each have multiple online presences, and from them, you can learn a lot about genes, B vitamins and methylation.
2. Learn About Your Genes
Get a full genetic test done to gather your raw data. There are many services available that offer genetic testing. If you already have an Ancestry or 23andMe test, you can download and utilize that raw data.
Run your raw data through a program that can interpret your genetic makeup, identifying which genes may impact your health and suggesting remedial actions. I haven’t fully explored these services, but many exist. Some may be beyond my budget, but one that has been recommended in a Facebook MTHFR support group is Living with MTHFR. I plan to engage with at least this level of support, as I’ve realized I can’t tackle everything on my own.
3. Get Support
You don’t have to go through this alone. Consider recruiting ongoing personalized support from a professional in genetics to help you implement your findings.
If hiring a professional isn’t feasible, learn how to use tools like ChatGPT or other AI to double-check your conclusions and potential actions. AI can be a helpful resource when personalized support is out of reach.
Much of your progress can be gauged by how you feel, but tracking ‘numbers’ is also valuable. Try to find a friendly healthcare provider, ideally knowledgeable in genetics and methylation, who can order tests to help you monitor your health. Regular checks of your homocysteine levels and other relevant biomarkers can provide insights into how your body is managing methylation, informing any necessary adjustments to your diet and supplements.
4. Learn How to Use Diet to Manage Your Methylation Issues
We’ve all received plenty of dietary advice over the years, but now you have a personal understanding of why some foods and diets work better for you than others. Since no one shares your exact genetic makeup, your nutritional needs are unique.
Focus on a diet rich in methyl donors and nutrients that support methylation, such as:
Folate Sources: Leafy greens, legumes, and liver.
Choline Sources: Eggs, meat, fish, and beans.
Betaine Sources: Beets, spinach, and whole grains.
B Vitamins: Especially B6, B12, and riboflavin, which are essential for the methylation cycle.
5. Develop a Personalized Supplement Regimen
For those with MTHFR variants, supplementation is often a beneficial approach, but the best regimen for you depends on your specific genetic variants, including MTHFR, COMT, and PEMT.
5-MTHF: Instead of relying solely on folate, some people find that taking 5-MTHF supplements can help bypass the MTHFR bottleneck. Start with low doses and monitor how you feel.
Methylation Support: Supplements like betaine (TMG), choline, and B vitamins can support methylation. Start slowly and observe any changes in your symptoms to avoid overdoing it.
6. Develop a Personalized Detox Regimen
Regardless of our MTHFR variants, we all face some level of detox compromise. We must figure out the safest and most sustainable ways to detox for the long haul. Given our toxic environment, we can’t expect our bodies to keep pace with the required detoxification, even with lifestyle changes.
I prefer to avoid chemical detox agents, which make me uneasy. I don’t like the idea of using a poison to detox a poison. Instead, I stick to plant-based detox agents, which still need careful consideration:
modified citrus pectin (for heavy metal detox and cancer management)
activated silica (for aluminium detox)
fulvic/humic acid (as a general mineral supplement and general detox aid)
7. Develop the Right Mental Approach
Focus on balance, not perfection. There is no way of knowing how much of what you need to eat or supplement- it is all guesswork, and so you will have to engage in a lot of careful trial and error to get it right:
Instead of striving for a “perfect” level of methylation, aim for a balance that suits your body. This often involves trial and error, guided by your feelings and health markers.
Keep track of how you feel with different dietary changes and supplements. Symptoms like mood swings, fatigue, or cognitive changes can offer clues about whether your methylation levels are too high or too low.
Allow time: I have set up a range of tests that my local GP is willing to order. These tests will give me some kind of feedback on whether my overall health is improving over time, and whether my methylation levels (and mitchondrial health) appear to be improving. The difficulty is that we have no direct measures for methylation levels, and we will only see the results of our efforts over time, as our body uses its improved methylation capacity to choose what it chooses to heal. It may not be possible to see a direct link between taking steps to improve methylation (which we cannot measure) and improved health outcomes. So we need to take time. I have given a nominal 12 months during which time I will have regular testing of a number of health indicators, and during which time I will do what I can to improve my methylation. Only by the end of the 12 months will my doctor and I know if anything I have been doing is worthwhile, when we see measurable downstream health improvements.
8. Look for Ways of Measuring Improved Health
I asked ChatGPT whether my smart watch can give me any clues on my methylation status and surprisingly, it seems it can, or at least it can help me determine if my overall metabolic health is improving, and that is not going to happen if methylation does not improve.
Heart Rate Variability (HRV) measures the variation in time between heartbeats and is a key indicator of autonomic nervous system balance (parasympathetic vs. sympathetic). Low HRV can indicate chronic stress, poor recovery, or an overactive sympathetic nervous system, which might reflect issues with methylation, particularly if your COMT gene is slow (which is linked to slower breakdown of stress hormones like dopamine and norepinephrine). Higher HRV reflects better vagal tone, metabolic flexibility, and stress resilience, possibly indicating a more balanced methylation process.
Sleep Tracking (especially Deep Sleep). Many smartwatches track light, deep, and REM sleep stages. Deep sleep is particularly important for body repair, detoxification, and cellular regeneration, all of which are influenced by proper methylation. A lack of deep sleep, could indicate stress, neurotransmitter imbalances, or energy metabolism issues, potentially linked to methylation dysfunction. Sleep disruption can reduce methylation efficiency, so improving sleep might also help balance methylation processes.
Blood Oxygen (SpO2) measures oxygen saturation in the blood, which can be linked to cardiovascular and respiratory health. Lower blood oxygen levels over time may be linked to reduced mitochondrial function or oxidative stress, which can be affected by poor methylation. Mitochondrial function requires methylation for the synthesis of CoQ10 and ATP, both essential for energy production.
Resting Heart Rate (RHR) is a measure of how efficiently your cardiovascular system functions at rest. a consistently high resting heart rate could be a sign of chronic stress or metabolic dysregulation, potentially linked to methylation issues (e.g., slow breakdown of stress hormones). A balanced methylation cycle supports heart and nervous system health, so any sustained deviation in RHR could signal an imbalance.
Stress Tracking (via Electrodermal Activity or HRV). Some smartwatches track stress levels based on changes in heart rate or skin conductivity (electrodermal activity). High stress could reflect sympathetic dominance and neurotransmitter imbalances, which could be associated with methylation issues, particularly if methylation is underactive and unable to regulate stress hormones properly. Since methylation plays a role in breaking down stress-related neurotransmitters (like dopamine and epinephrine), higher stress readings might signal methylation bottlenecks.
Body Temperature (if available): Fluctuations in body temperature can reflect changes in metabolic rate. Poor methylation can sometimes slow down metabolism, affecting how the body generates energy and regulates temperature. Low body temperature could hint at sluggish metabolism, while higher body temperature might indicate hyperactivity or inflammation.
Calories Burned (Metabolic Rate). Smartwatches calculate calories burned based on heart rate, movement, and sometimes body composition. Tracking calorie burn can give insight into your overall metabolic rate. If your metabolism seems unusually low despite physical activity, this could be a sign of mitochondrial inefficiency, which may be connected to methylation issues. On the other hand, if you burn an unusually high number of calories at rest, this might reflect metabolic overactivity or stress, potentially linked to sympathetic nervous system overdrive.
VO2 Max (Cardiorespiratory Fitness) estimates the maximum amount of oxygen your body can use during intense exercise and is a key indicator of cardiovascular fitness and metabolic health. Mitochondrial function and energy production are crucial for VO2 max, both of which are impacted by methylation. Poor methylation can impair mitochondrial efficiency, potentially leading to a lower VO2 max. Conversely, improving methylation might lead to better oxygen utilization and metabolic efficiency.
Blood Glucose (if available): Some devices monitor blood glucose levels throughout the day. Blood glucose regulation is closely tied to metabolic health. Poor glucose control may indicate metabolic syndrome or insulin resistance, which could be related to methylation problems, particularly if the methylation cycle isn't supporting normal glucose metabolism.
Can you help?
I intend to keep writing of my journey through this rather strange time in my life, and for this planet. As soon as I know, I will share with you, what does and does not work to exorcise this bio-weapon from our bodies and take back some level of control of our lives.
If you think my writings are worthwhile, you can help me get more subscriptions and donations by sharing this article on other social media platforms, and by re-stacking this article in Substack Notes.
Or make a PayPal donation here to help with ongoing medical costs. Every little bit helps. The last donations, thank you, paid for a red light to help me find restful sleep and experimental nitric oxide gummies to see if increasing nitric oxide levels improves my overall health (it does). I now have a non-intrusive blood glucose monitor on the way and my second order of AgeMate, a supplement to support mitchondrial health and methylation.




This is incredibly good, accurate information, condensing a very complex subject in understandable language. Thank you.
Whatever I got poisoned with in 2022 it did something to the gene in question and now I am on a methylation diet and I kind of figured out myself because it came across that gene info in 2020 in researching my high blood pressure and being allergic to all the blood pressure medicine at times I felt like I was going to die, and I would drive myself to the hospital.
For supplementation, I’m taking a premier vitamin product called phyto methylate
Thank you for all this valuable information.